
Large corporations are fond of saying that they are all about giving customers what they want. But what happens when the product, though legal, is harmful or addictive? Should companies be allowed to satisfy consumer demand, no matter what the consequences?
Some high-profile settlements announced in recent weeks show that, as far as state attorneys general are concerned, there should be much stricter controls on the marketing of dangerous products and that corporations should be heavily penalized for abuses.
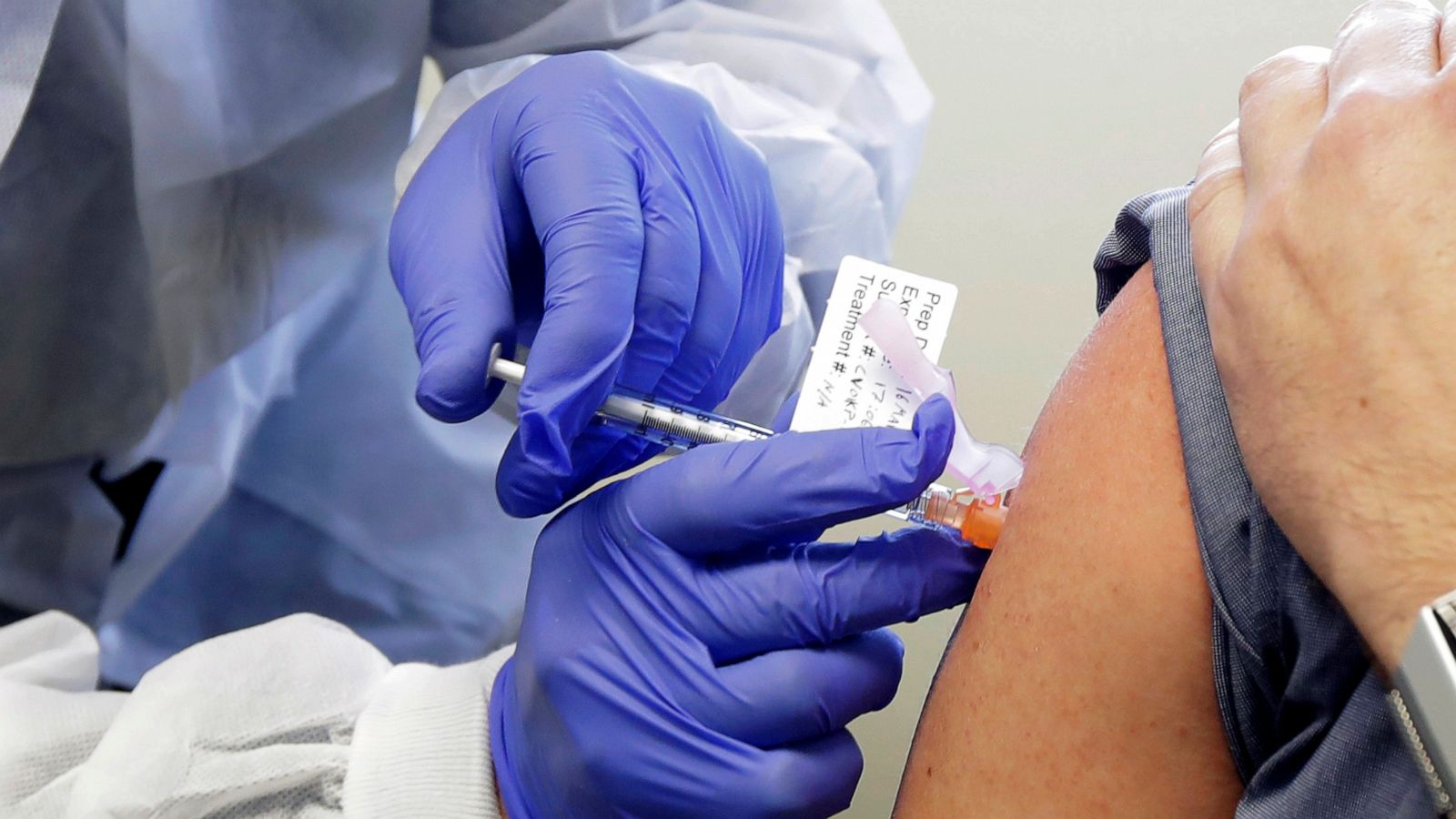
The most recent case involves JUUL Labs, which just agreed to pay $438 million to resolve multistate litigation accusing it of improperly marketing vaping products to minors. The announcement of the settlement, reach with a bipartisan group of 34 states and territories, alleged that JUUL “relentlessly marketed to underage users with launch parties, advertisements using young and trendy-looking models, social media posts and free samples. It marketed a technology-focused, sleek design that could be easily concealed and sold its product in flavors known to be attractive to underage users. JUUL also manipulated the chemical composition of its product to make the vapor less harsh on the throats of the young and inexperienced users. To preserve its young customer base, JUUL relied on age verification techniques that it knew were ineffective.”
Earlier this summer, groups of state AGs announced several massive settlements with companies involved in the production and marketing of opioids. Teva Pharmaceuticals agreed to pay up to $4.25 billion to state and localities to settle allegations that it promoted two powerful fentanyl products designed for cancer patients to others while downplaying the risks of addiction. According to the AGs’ press release, Teva’s actions included “encouraging the myth that signs of addiction are actually ‘pseudoaddiction’ treated by prescribing more opioids.”
Several days later, Allergan agreed to pay up to $2.4 billion to settle a similar multistate case alleging the company had “deceptively marketed opioids by downplaying the risk of addiction, overstating their benefits, and encouraging doctors to treat patients showing signs of addiction by prescribing them more opioids.” Allergan was also accused of failing to maintain effective controls to prevent the diversion of opioids into improper channels.
And a couple of weeks after that, Endo International agreed to pay $450 million in yet another multistate lawsuit stemming from accusations of deceptive marketing. In Endo’s case, this involved allegations that it “falsely peddled its opioids as abuse-deterrent with deadly consequences.” Under the weight of this and other litigation, Endo has filed for bankruptcy.
The theme running through all these cases is the tendency of corporations, obsessed with the desire to increase sales, to engage in shockingly unethical behavior. Both the slick techniques of JUUL to lure young vapers and the seemingly scientific claims of the opioid producers to reassure pain patients demonstrate an apparent willingness to use deceit and manipulation to push dangerous products on vulnerable populations. The monetary penalties, however large, and promises to end the abuses hardly seem a sufficient penalty for the harm caused by this behavior.








You must be logged in to post a comment.